Lab-Aids: Introduction to Conductivity Kit
All materials can be classified as a conductor, insulator or semi-conductor. Materials are classified according to their resistance to the flow of current. A conductor is a substance that is capable of carrying an electric charge from one point to another. An insulator blocks the flow of current. Semi-conductors are neither. Their conductivity lies in between the two.
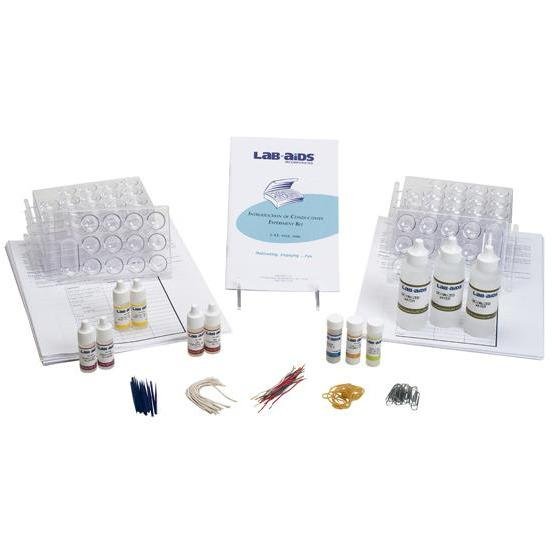
In Part 1 of this interesting Lab-Aids, students test several common objects to determine if they are conductors or insulators. In Part 2, The students test electrical conductivity in aqueous solutions.They test an acid, base, salt, alcohol and a hydrocarbon. This kit is an excellent introduction for students in physical science, general science and introductory chemistry courses. Conductivity indicators are required for these activities, but are not included with this kit. We recommend the LAB-AIDS‚® Conductivity Indicator. This kit is packaged complete for 30 students. REQUIRES CONDUCTIVITY INDICATORS.
Scientific Concepts:
- Explores electrical conductivity
- Develop principles of conductors and insulators
- Develop principles of electrolytes and non-electrolytes using acids, bases, salts, alcohols, and hydrocarbons
- Test a variety of common materials for their conductivity characteristics
- Differentiate between strong, weak, and non-conductors of electricity
Content List:
- 1 Teacher's Guide with MSDS
- 30 Student Worksheets and Guides
- 15 Chemplates‚® with measuring and mixing spatulas
- 1 String, cut, pkg
- 1 Paper clips, metal, pkg
- 1 pics, plastic, pkg
- 1 Rubber bands, pkg
- 1 Insulation copper wire, strips, pkg
- 2 Bottles of hydrochloric acid, 6M, 1/4 oz
- 2 Bottles of denatured alcohol, 1/4 oz
- 2 Bottles of glacial acetic acid, 1/4 oz
- 3 Bottles of deionized water, 2 oz
- 1 Vial of calcium hydroxide
- 1 Vial of sodium chloride crystals
- 1 Vial of sucrose crystals, solid
Classroom Planning:
- To complete this kit requires one ~50 minute periods
- Number of students: 30
You may also like
More from Middle School Science
Recently viewed