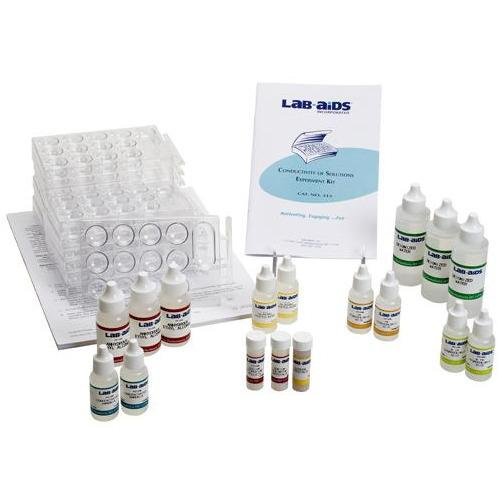
Lab-Aids: Conductivity of Solutions Experiment Kit
Using LAB-AIDS‚® Conductivity Indicators, students study the principles of ionization by differentiating between strong electrolytes, weak electrolytes and non-electrolytes. They also learn to differentiate between strong and weak acids and strong and weak bases. Performing a series of fun activities, students observe and discover dramatic results which lead to a better understanding of the principles of ionization. This Lab-Aid is designed for use by students in introductory chemistry courses and higher. Conductivity indicators are required for these activities but are not included. We recommend the LAB-AIDS‚® Conductivity Indicator. This kit is packaged for use with 30 students.
Scientific Concepts:
- Study principles of ionization
- Understand strong and weak electrolytes
- Differentiate between strong and weak acids and bases
Content List:
- 1 Teacher's Guide with MSDS
- 30 Student Worksheets and Guides
- 15 Chemplates‚® with measuring spatula
- 3 Drop control bottles of Ethyl alcohol, 1 oz
- 3 Drop control bottles of Hydrochloric acid, 0.1M, 1/2 oz
- 3 Drop control bottles of Hydrochloric acid, 1M, 1/2 oz
- 2 Drop control bottles of Ammonia, concentrated, 1/2 oz
- 2 Drop control bottles of Glacial Acetic acid, 1/2 oz
- 2 Drop control bottles of Deionized water, 2 oz
- 3 Drop control bottles of Anydyrous ethyl alcohol
- 1 Vial of Sodium Chloride crystals
- 1 Vial of Sodium Hydroxide pellets
- 1 Vial of Sucrose crystals
Classroom Planning:
- To complete this kit requires one ~50 minute periods
- Number of students: 30