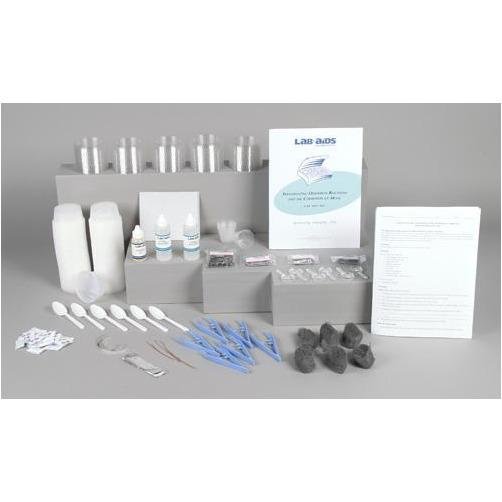
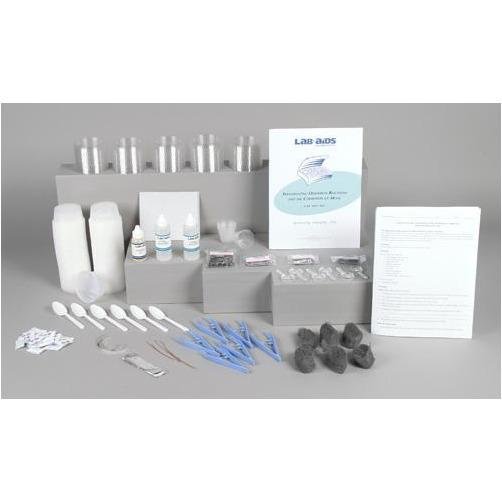
Lab-Aids: Investigating the Chemistry of Corrosion Kit
This is a two-part investigation into the chemical concepts that underlie corrosion and our attempts to inhibit it. In the laboratory portion of this activity, students investigate the corrosion of iron, steel, aluminum, and zinc metal. They investigate the impact of factors such as salinity, coatings, and contact with other metals on rust and corrosion. Students also complete a reading that describes some conditions that affects the rate of corrosion of iron and steel and several chemical principles associated with these conditions. The activity concludes with a discussion of the impact of corrosion on materials and the environment. Developed by SEPUP.
Scientific Concepts:
- Substances react chemically in characteristic ways with other substances to form new substances (compounds) with different characteristic properties
- Metals are a group of substances that react in similar ways. Corrosion is one type of reaction that affects metals
- Metal corrosion is an example of an oxidation-reduction (redox) reaction, which involves the transfer of electrons
- Students summarize results of an investigation and form a logical argument about the cause-and-effect relationships in the investigation
Content List:
- 1 Teacher's Guide with MSDS
- 28 Student Worksheets and Guides
- 100 3.25-ounce plastic cups and lids
- 48 Iron nails
- 24 Packets of salt
- 12 Stainless steel nails
- 12 Aluminum nails
- 12 Galvanized nails
- 12 Pieces of steel wool
- 6 10 cm (4) pieces of 20-gauge copper wire
- 6 10 cm (4) pieces of magnesium ribbon
- 6 10 cm x 2 cm (4 x 1) pieces of aluminum foil
- 6 30-mL graduated cups
- 6 Tweezers
- 6 Plastic spoons
- 6 Pieces of sand paper
- 6 Magnifying lenses
- 2 Denatured ethyl alcohol, 60-mL bottle
- 1 Cooking oil, 30-mL bottle
Classroom Planning:
- To complete the two activities in this kit requires two to three ~50-minute class periods.
- Number of students: 24
You may also like
More from Best selling products
Recently viewed