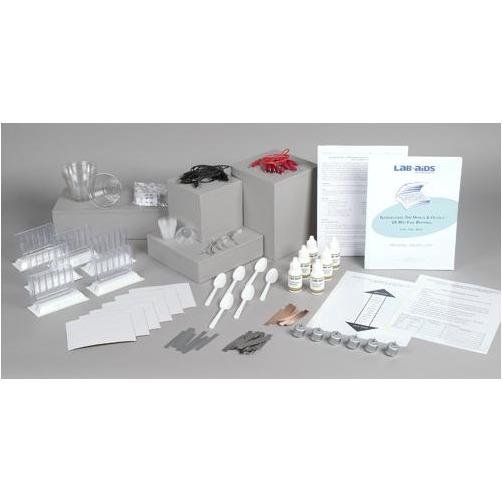
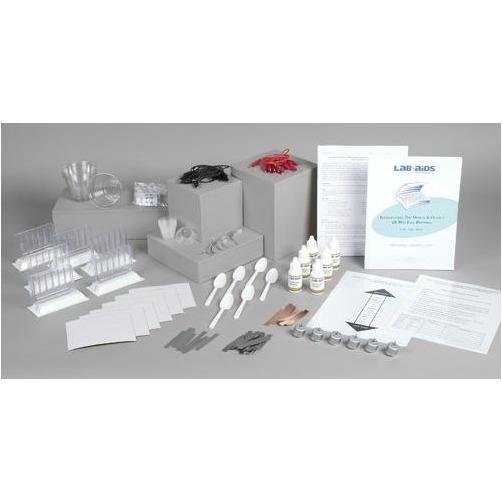
Lab-Aids: Investigating the Design & Output of Wet Cell Batteries Kit
This kit introduces students to the concept of energy conversion through the study of a simple electrochemical cell connected to an electric motor. Electrochemical cells are the basis for batteries and contain metals and other chemicals with stored chemical potential energy. Students design and conduct an experiment that uses the unique LAB-AIDS‚® Wet Cell Chamber to determine which combination of different metals works best to convert the potential chemical energy into electric energy. Their results allow them to rank the metals tested in order of reactivity. They also investigate how the distance between the metals, the direction of current flow, and the amount of metal surface area impacts the electrical output of the wet cell. Developed by SEPUP.
Scientific Concepts:
- Chemicals contain stored energy that can be released during a reaction
- Batteries, or electrochemical cells, transform chemical potential energy into electric energy
- Students design and conduct a scientific investigation
- Students use appropriate tools and techniques to gather, analyze, and interpret data
- Students use evidence to develop descriptions, explanations, predictions, and models
Content List:
- 1 Teacher's Guide with MSDS
- 28 Student Worksheets and Guides
- 30 Table Salt
- 12 Copper strips
- 12 Iron strips
- 12 Magnesium strips
- 12 Zinc strips
- 6 Electric motors
- 6 Wet Cell Chambers SEPUP‚®
- 6 Black wire leads with alligator clips
- 6 3% Hydrogen Peroxide solution, 30-mL dropper bottles
- 6 Graduated cups, 30-mL
- 6 Plastic cups, 270-mL (9-oz.)
- 6 Sandpaper, small pieces
- 6 Red wire leads with alligator clips
- 6 Plastic spoons
- 1 Transparency 1: Sample Procedure and Data Table for Part B
- 1 Transparency 2: Metals Activity Charta
Classroom Planning:
- To complete this kit requires one to two ~50-minute class periods
- Number of students: 24
You may also like
More from All STEMfinity Brands
Recently viewed